Abstract
The ELN recommendations for diagnosis and management of AML in adults, authored by a panel of international experts, have become an important tool to assess pts' prognosis and guide treatment decisions. Following advances in our understanding of the molecular landscape of AML, several gene mutations were added as criteria for assigning pts to genetic risk categories in the 2017 ELN classification.
The aim of our study was to apply the 2017 ELN risk groups to a large cohort of 864 AML pts aged <60 years who were similarly treated with intensive cytarabine/daunorubicin based chemotherapy on Cancer and Leukemia Group B/Alliance protocols. In addition, we sought to identify novel gene markers that could refine the ELN classification.
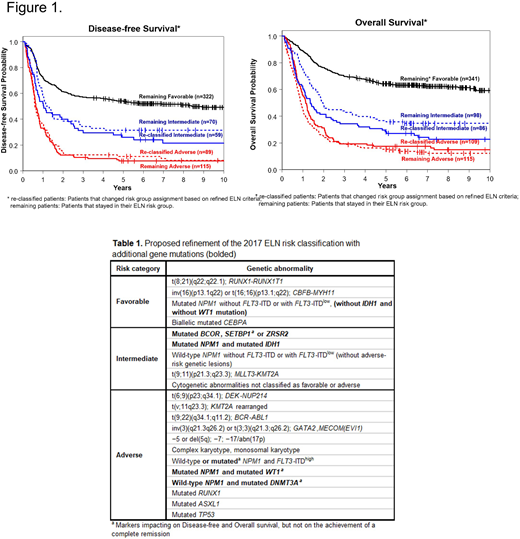
Using the 2017 ELN genetic risk-stratification criteria, we assigned 49% of pts to the Favorable-risk group, whereas 22% and 29% of pts belonged to the Intermediate- and Adverse-risk groups, respectively. The complete remission (CR) rate of Favorable-risk pts was 92%, compared with 77% and 48% CR rates of Intermediate-risk and Adverse-risk pts. Fifty-two percent of Favorable-risk pts were disease-free and 63% were alive 3 years after diagnosis, compared with the respective 3-year rates of 22% and 32% for pts belonging to the Intermediate-risk group, and only 10% and 19%, respectively, for pts classified in the Adverse-risk group. Using a targeted 80-gene sequencing panel, we detected 2,354 mutations, with a median of 3 per pt. The frequencies of specific mutations differed between the ELN risk groups. We performed multivariable outcome analyses for the achievement of CR, disease-free (DFS) and overall (OS) survival within each of the ELN groups. Within the Favorable-risk group MVA analyses, the presence of WT1 mutations associated with lower CR rates (P=0.02). Mutations in BCOR (P=0.02), IDH1 (P=0.04), KIT (P<0.001), PTPN11 (P=0.01) and SETBP1 (P=0.02) associated with a shorter DFS; mutated BCOR (P=0.02), IDH1 (P=0.006), SETBP1 (P=0.004), WT1 (P<0.001) and ZRSR2 (P=0.002) associated with a shorter OS. In the Intermediate-risk group MVA analyses, mutations in WT1 associated with lower CR rates (P=0.02). Mutations in DNMT3A and WT1 associated with shorter DFS (DNMT3A, P<0.001; WT1, P=0.01) and OS (DNMT3A, P<0.001; WT1, P=0.005). In the Adverse-risk group MVA analyses, IDH2 mutations associated with lower CR rates (P=0.05). NRAS mutations associated with shorter DFS (P<0.001) and OS (P=0.008). In addition, IDH2- (P=0.03) and TET2-mutated pts (P=0.009) had shorter OS than pts without these mutations. Among FLT3-ITDlow pts, those harboring NPM1 mutation had higher CR rates (P<0.001) and longer OS (P=0.001), but not DFS (P=0.16), than pts with wild-type (wt) NPM1. Within the FLT3-ITDhigh cohort, pts with an NPM1 mutation had higher CR rates than those without (P<0.001). However, there was no difference in either DFS or OS between NPM1 mutated and NPM1 wt pts, indicating that the negative prognostic impact of FLT3-ITDhigh may outweigh the positive prognostic impact of NPM1 mutations with respect to those survival endpoints. We also noted that the negative prognostic impact of WT1 mutations was worsened by co-occurring NPM1 mutations. Similarly, the impact of IDH1 mutations within the Favorable group was influenced by NPM1 mutations, with IDH1/NPM1 co-mutated patients having inferior survival. Based on the results of our multivariable analyses, as well as co-occurring mutations, we provide a possible refinement of the ELN genetic risk stratification in Table 1. DFS and OS of the re-classified pts were comparable to DFS and OS of the original ELN groups (Figure 1). Importantly, the re-classification also changed the percentages of AML patients assigned to specific risk groups, reducing the Favorable-risk group from 49% to 39% and increasing the Adverse-risk group from 29% to 38%, with the Intermediate-risk group remaining almost the same (23% vs 22%). In summary, our study provides a comprehensive analysis of genetic prognostic markers in younger AML pts treated with standard chemotherapy within the framework of the ELN risk classification. Furthermore, we identified several combinations of co-occurring mutations with prognostic significance. Our results provide a refinement of the 2017 ELN risk classification, and identify additional pts that may need more intensive treatment early on.
Support: U10CA180821, U10CA180882, U10CA180861, U24CA196171; ClinicalTrials.gov
Mims:Abbvie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy. Kolitz:Magellan Health: Consultancy, Honoraria. Powell:Rafael Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal